

Despite associated improvements in the survival rate, however, non−HLA-identical hematopoietic stem-cell transplantation has a number of drawbacks. 2,3 Hematopoietic stem-cell transplantation is a lifesaving therapy. This condition is characterized by the complete lack of T cells and natural killer cells, whereas B cells are present.
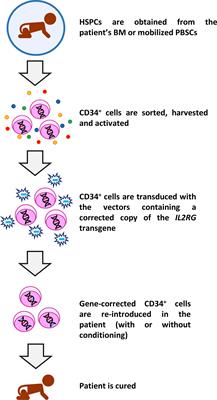
1 Naturally occurring mutations in IL2RG are responsible for X-linked severe combined immunodeficiency (SCID-X1) disease. The cytokine receptor common γ chain, which is encoded by the interleukin-2 receptor subunit gamma ( IL2RG) gene, is a critical functional component of the receptors for interleukin-2, interleukin-4, interleukin-7, interleukin-9, interleukin-15, and interleukin-21. (Funded by INSERM and others.) Introduction This treatment is associated with a risk of acute leukemia. Gene therapy may be an option for patients who do not have an HLA-identical donor for hematopoietic stem-cell transplantation and for whom the risks are deemed acceptable. ConclusionsĪfter nearly 10 years of follow-up, gene therapy was shown to have corrected the immunodeficiency associated with SCID-X1. Correction of the immunodeficiency improved the patients' health. The T-cell−receptor repertoire was diverse in all patients. Sustained thymopoiesis was established by the persistent presence of naive T cells, even after chemotherapy in three patients. Seven patients, including the three survivors of leukemia, had sustained immune reconstitution three patients required immunoglobulin-replacement therapy. Transduced T cells were detected for up to 10.7 years after gene therapy. However, acute leukemia developed in four patients, and one died. Gene therapy was initially successful at correcting immune dysfunction in eight of the nine patients. ResultsĮight patients were alive after a median follow-up period of 9 years (range, 8 to 11). We assessed clinical events and immune function on long-term follow-up. The nine patients, who lacked an HLA-identical donor, underwent ex vivo retrovirus-mediated transfer of γ chain to autologous CD34+ bone marrow cells between 19. We reviewed long-term outcomes after gene therapy in nine patients with X-linked severe combined immunodeficiency (SCID-X1), which is characterized by the absence of the cytokine receptor common γ chain. The outcomes of gene therapy to correct congenital immunodeficiencies are unknown. The most trusted, influential source of new medical knowledge and clinical best practices in the world.
GENE THERAPY FOR SCID LICENSE
Information and tools for librarians about site license offerings. Valuable tools for building a rewarding career in health care. The authorized source of trusted medical research and education for the Chinese-language medical community. The most advanced way to teach, practice, and assess clinical reasoning skills. Information, resources, and support needed to approach rotations - and life as a resident. The most effective and engaging way for clinicians to learn, improve their practice, and prepare for board exams. NEW! Peer-reviewed journal featuring in-depth articles to accelerate the transformation of health care delivery.Ĭoncise summaries and expert physician commentary that busy clinicians need to enhance patient care.
GENE THERAPY FOR SCID TRIAL
NEW! A digital journal for innovative original research and fresh, bold ideas in clinical trial design and clinical decision-making.


 0 kommentar(er)
0 kommentar(er)
